What is the bond order and magnetic behavior of the molecule?
Carbon monoxide CO is a heteronuclear diatomic molecule, meaning it consists of two different atoms. In this case, carbon C and oxygen O . To calculate the molecular orbital energy diagram and electronic configuration, we will use the Molecular Orbital Theory MOT for heteronuclear diatomic molecules.
The atomic orbitals of carbon C are 1s², 2s², and 2p², while the atomic orbitals of oxygen O are 1s², 2s², and 2p⁴. In the formation of CO, we will focus on the valence orbitals, which are the 2s and 2p orbitals.
The molecular orbitals formed from the combination of 2s orbitals are σ 2s and σ* 2s . The molecular orbitals formed from the combination of 2p orbitals are σ 2pz , π 2px , π 2py , σ* 2pz , π* 2px , and π* 2py .
The molecular orbital energy diagram for CO is as follows:
1. σ 2s
2. σ* 2s
3. σ 2pz
4. π 2px and π 2py
5. σ* 2pz
6. π* 2px and π* 2py
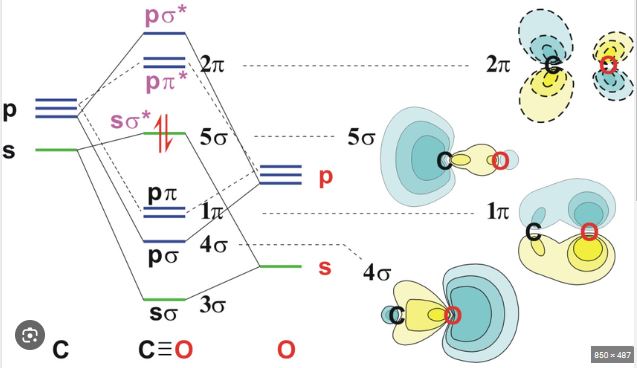
Now, we will fill the molecular orbitals with the 10 valence electrons from carbon and oxygen 4 from C and 6 from O :
1. σ 2s – 2 electrons
2. σ* 2s – 2 electrons
3. σ 2pz – 2 electrons
4. π 2px and π 2py – 4 electrons 2 in each orbital
5. σ* 2pz – 0 electrons
6. π* 2px and π* 2py – 0 electrons
The electronic configuration of CO is: σ 2s ², σ* 2s ², σ 2pz ², π 2px ², π 2py ².
To calculate the bond order, use the formula:
Bond order = number of electrons in bonding orbitals – number of electrons in antibonding orbitals / 2
Bond order = 8 – 2 / 2 = 6 / 2 = 3
The bond order of CO is 3, indicating a strong triple bond between the carbon and oxygen atoms.
As for the magnetic behavior, all the molecular orbitals are filled with paired electrons, so CO is diamagnetic, meaning it does not have unpaired electrons and is not attracted to a magnetic field.

