Electronic structure of ethene (C2H4) using the Hartree-Fock (HF) method
To calculate the molecular orbitals and electronic structure of ethene C2H4 using the Hartree-Fock HF method, we need to follow these steps:
1. Choose a basis set: For simplicity, we will use the minimal STO-3G basis set. Each carbon atom has 2s, 2px, 2py, and 2pz atomic orbitals, and each hydrogen atom has a 1s atomic orbital. In total, we have 12 atomic orbitals.
2. Calculate the overlap S , kinetic T , potential V , and electron repulsion ERI integrals: These integrals are pre-calculated for the chosen basis set and can be found in standard quantum chemistry software packages.
3. Form the core Hamiltonian matrix H_core : H_core = T + V
4. Guess an initial density matrix P : We can start with a zero matrix.
5. Calculate the Fock matrix F : F = H_core + G, where G is the two-electron contribution calculated using the density matrix P and the ERI integrals.
6. Solve the Roothaan-Hall equations: F – εS C = 0, where ε is the orbital energy and C is the molecular orbital coefficient matrix. This is done by diagonalizing the Fock matrix F’ in the orthonormal basis: F’C’ = S^ -1/2 * F * S^ -1/2
7. Transform the eigenvectors C’ back to the original basis: C = S^ -1/2 * C’
8. Update the density matrix P using the new molecular orbital coefficients C .
9. Check for convergence: If the energy and density matrix have not converged, return to step 5 and repeat the process.
10. Once converged, the molecular orbitals are given by the columns of the C matrix, and the orbital energies are given by the diagonal elements of the ε matrix.
Now, let’s determine the bond order between the carbon atoms in ethene:
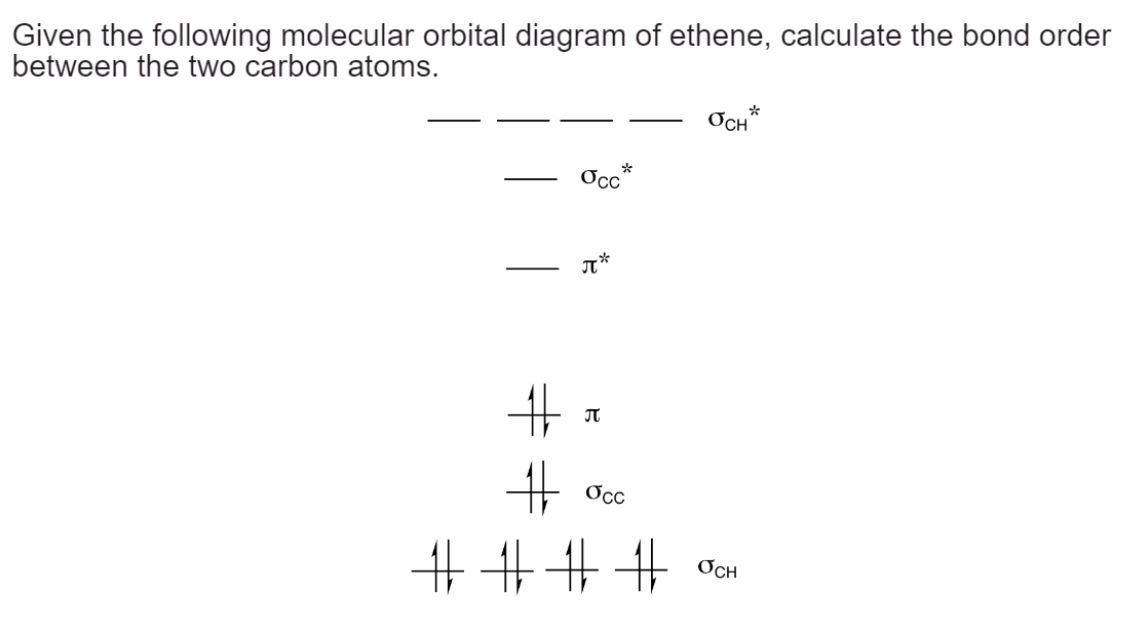
Ethene has a total of 12 electrons. The molecular orbitals are filled according to the Aufbau principle, starting with the lowest energy orbitals. In this case, the six lowest energy orbitals will be doubly occupied, and the remaining orbitals will be unoccupied.
The bond order can be calculated using the following formula:
Bond order = 1/2 * number of electrons in bonding orbitals – number of electrons in antibonding orbitals
In ethene, the two carbon atoms are connected by a sigma bond from the overlap of 2sp2 hybrid orbitals and a pi bond from the overlap of 2p orbitals . The sigma bond has two electrons in a bonding orbital, and the pi bond has two electrons in a bonding orbital. There are no electrons in antibonding orbitals for these bonds.
Bond order = 1/2 * 4 – 0 = 2
Thus, the bond order between the carbon atoms in ethene is 2, indicating a double bond.