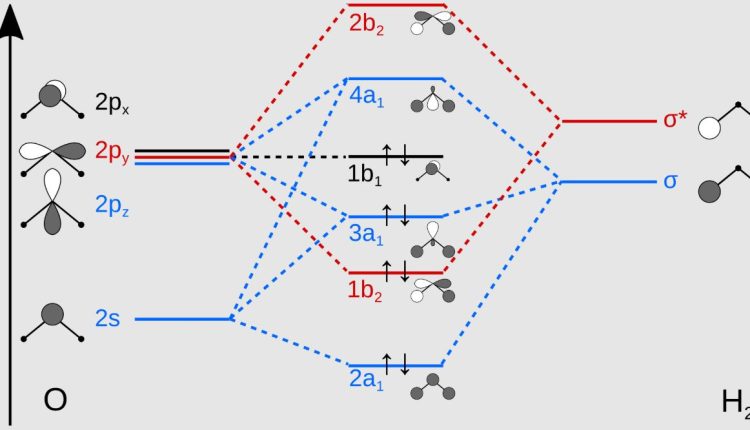
H2O molecule using the method of linear combination of atomic orbitals (LCAO) theory.
To calculate the molecular orbital energy diagram and electronic structure of the H2O molecule using the LCAO method, we first need to consider the atomic orbitals of the constituent atoms, hydrogen H and oxygen O .
1. Atomic orbitals:
– Hydrogen has one 1s orbital.
– Oxygen has the following orbitals: 1s, 2s, and three 2p orbitals 2px, 2py, 2pz .
2. Formation of molecular orbitals:
– Two hydrogen 1s orbitals combine with the oxygen 2s and one of the 2p orbitals let’s say 2pz to form three molecular orbitals: one bonding σ , one non-bonding n , and one antibonding σ* .
– The remaining two oxygen 2p orbitals 2px and 2py remain non-bonding orbitals.
3. Filling electrons in molecular orbitals:
– H2O has a total of 10 electrons 2 from each hydrogen and 6 from oxygen .
– According to the aufbau principle, electrons fill the molecular orbitals in increasing order of energy.
– The filling order is: σ 2 electrons , n 2 electrons , 2px 2 electrons , 2py 2 electrons , and σ* 2 electrons .
4. Molecular orbital energy diagram:
– The energy levels of the molecular orbitals can be represented as follows from lowest to highest energy : σ < n ≈ 2px ≈ 2py < σ*.
– The diagram shows the bonding σ , non-bonding n, 2px, 2py , and antibonding σ* molecular orbitals, with the number of electrons in each orbital indicated.
5. Electronic structure:
– The electronic structure of the H2O molecule can be represented as: σ ² n ² 2px ² 2py ² σ* ².
In summary, the molecular orbital energy diagram and electronic structure of the H2O molecule using the LCAO method show one bonding, three non-bonding, and one antibonding molecular orbitals, with a total of 10 electrons distributed among them.